Machine learning accelerates R&D for key class of therapeutics
- Machine learning enabled improvements in product yield by helping to reduce critical impurities
- Automated process review achieved a 90% reduction compared to manual interpretation time for impurity analysis
- The ML model offered unique insights that help to overcome process limitations
- Alchemite™ identified sequence-specific parameters to optimize synthesis processes.

Summary
Researchers at the CPI Medicines Manufacturing Innovation Centre collaborated with Intellegens in a two-year project to develop and validate digital tools, based on the Alchemite™ machine learning platform, that can accelerate oligonucleotide process development. Supported by Innovate UK and guided by input from six leading pharma and biotech research organizations, the project addressed key challenges in the commercialization of oligonucleotides, a significant emerging class of therapeutics. Variability and impurities introduced during solid-phase oligonucleotide synthesis (SPOS) remain a significant barrier to robust, economic manufacturing. When impurities emerge early, downstream cleavage and deprotection (C&D), purification, and analytics become slower and costlier.
The project developed tools to ease the aggregation of data from oligonucleotide synthesis and analytics and to train machine learning (ML) models using this data. The models were then applied to two previously unseen SPOS scenarios. In the first scenario, the oligonucleotide sequence was known. In the second, there was no prior knowledge of the oligonucleotide sequence. In both cases, the machine learning was able to identify process parameters that achieved target properties for the synthesis, improving yields and reducing impurities, with many fewer cycles of experiment than would normally be required.
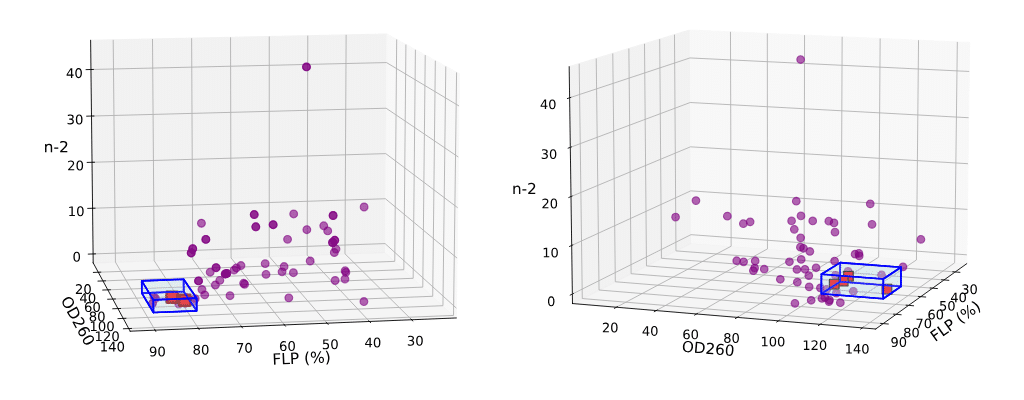
Alchemite™ demonstrated at least a 5% improvement in theoretical product yield by reducing critical impurities, fulfilling a key acceptance criterion.
Automated Process Review & Productivity: The system achieved a 90% reduction in manual interpretation time for impurity analysis, significantly improving productivity.
Reduced Expert Dependency: The software reduces the need for expert input through automation and guided decision-making.
Streamlines data capture and analysis: the ML model offers unique insights and helps to overcome current process limitations.
Optimizes synthesis: the solution identifies sequence optimal process parameters via virtual experiments.
Alchemite™ unified SPOS data, learned the complex mapping from synthesis conditions to outcomes, and delivered executable recommendations, closing the loop between experiment and optimization. Each run strengthens the model and reduces waste, enabling faster, cleaner, and more economical routes to commercial-grade oligonucleotides.
Barrie Cassey, Technology Lead at CPI, comments: “We’ve shown through this project how combining AI with process expertise can help exploit the potential of oligonucleotide therapeutics. Bringing oligonucleotides to market is costly, meaning very few companies are able to deliver the full potential of this life-saving and life-changing technology. The work
we’ve done in developing and validating the Alchemite™ tools will reduce development times and improve manufacturability of oligonucleotide therapeutics.”
The tools developed and validated through this project are now available commercially as the Alchemite™ for Oligonucleotide Manufacturing software solution.